ChemDAQ Monitoring System
Next Level Protection to Keep Your Teams Safe

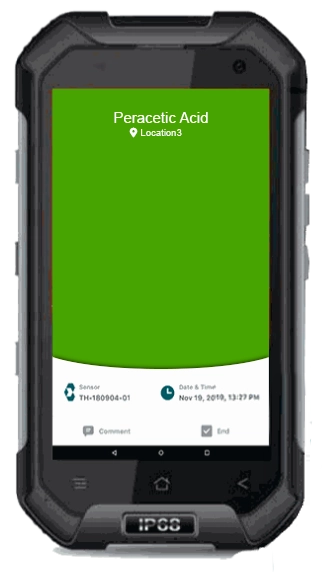

Safer Workplaces
Make sure your teams aren’t exposed to unsafe levels of ethylene oxide - EtO, hydrogen peroxide - H2O2 or peracetic acid - PAA by actively monitoring your workspaces. Proactive monitoring alerts you if your levels exceed acceptable limits so you can address the cause before it becomes a safety issue.

Reduced Risk
Working where there are unsafe levels of hazardous gases can lead to a myriad of health and safety issues. It also presents a significant liability risk, and can disrupt your operations. Be confident you are doing all you can to avoid over exposure for both your teams and your business.

Next Level Protection
ChemDAQ sets the standard for accurate and continuous gas detection of PAA, H2O2, and EtO vapors consistently outperforming other alternatives when it comes to helping you create a safer workplace. That’s why a who’s who of leading companies in a variety of industries rely on ChemDAQ.
Food and Beverage
Aseptic processing and packaging extend the shelf life of common food and beverages. To accomplish this, Peracetic Acid (PAA) and Hydrogen Peroxide (H2O2) are used primarily for their strong antimicrobial properties. Exposure to these chemical vapors during the production process can pose serious health and safety risks to workers.
Healthcare
Healthcare providers rely on high concentrations of Hydrogen Peroxide (H2O2), Peracetic Acid (PAA), and Ethylene Oxide (EtO) to sterilize instruments that come into contact with patients. Used to help prevent infection in patients, workers repeatedly exposed to these sterilant vapors can lead to numerous long term health problems.
Meat Processing
To comply with USDA food safety guidelines, beef, pork and poultry producers use Peracetic Acid (PAA) to kill harmful bacteria. PAA is used extensively on product, equipment and surfaces to eliminate food borne illnesses. Regulations are in place to protect workers from exposure to unsafe levels of PAA during the production process.
Medical Device
More than 50% of all medical devices used in the U.S. are sterilized with Ethylene Oxide. Workers are potentially exposed to high concentrations of Ethylene Oxide (EtO) during the sterilization process and in warehouse storage which can lead to a variety of health and safety risks.
Logistics
While an effective sterilizing agent, Ethylene Oxide (EtO) needs a long time to off-gas. Logistics providers and warehouse workers run the risk of exceeding OSHA's permissible exposure limit for Ethylene Oxide (EtO) emissions that accumulate during transport and in storage.
Get Started Today
To learn more about how active monitoring for unsafe levels of sterilization or antimicrobial chemicals can help you create a safer workplace, reduce risk, and provide next-level protection for your team, please fill out the form below, and a member of our team will contact you.
